New Mn-based cathode delivers record capacity and stability
GA, UNITED STATES, October 16, 2025 /EINPresswire.com/ -- Researchers have developed a manganese-based, cobalt-free lithium-excess layered cathode that significantly advances the performance of lithium-ion batteries. By employing an O2-type honeycomb structure, the material demonstrates high reversible capacity, long cycling stability, and improved thermal safety. This design achieves ~284 mAh g⁻¹ with an energy density of 956 Wh kg⁻¹, while maintaining about 70% capacity after 500 cycles in full cells. Unlike traditional cathodes prone to oxygen loss and structural degradation, the new composition stabilizes the oxygen redox process and suppresses phase transitions. These findings mark a critical step toward sustainable, high-capacity, and long-lasting lithium-ion batteries for next-generation applications.
The growing demand for high-performance lithium-ion batteries in electric vehicles, renewable energy storage, and portable electronics has highlighted the limitations of current cathode technologies. Conventional materials such as LiCoO₂ and Ni-rich oxides offer good capacity but suffer from cost, sustainability, and safety concerns. Mn-based oxides are more abundant and thermally stable but often face challenges including poor cycling performance and irreversible structural changes. Oxygen redox chemistry offers a pathway to boost capacity but is often hindered by oxygen release and phase transitions. Due to these issues, researchers need to explore cobalt-free, structurally stable cathodes that maximize both energy density and durability.
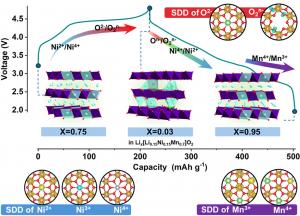
A research team from Sejong University and international collaborators reported a new lithium-excess cathode, Li₀.₇₅[Li₀.₁₅Ni₀.₁₅Mn₀.₇]O₂, in the journal eScience on July, 2025. The study demonstrates how this O2-type layered oxide, synthesized via ion-exchange, overcomes common challenges of oxygen redox instability. The novel material not only achieves exceptional energy density but also retains structural integrity during prolonged cycling. This innovation points to a sustainable, cobalt-free direction for lithium-ion batteries and offers new insights into balancing high performance with long-term reliability.
The newly developed cathode material features a distinctive O2-type layered framework with honeycomb ordering of transition metals. Using neutron diffraction, X-ray techniques, and advanced simulations, the researchers confirmed its structural stability and electrochemical mechanisms. The ion-exchange process from Na-based precursors yielded a highly ordered arrangement, enabling efficient lithium diffusion and reversible oxygen redox. Electrochemical tests revealed a discharge capacity of 284 mAh g⁻¹ and energy density of 956 Wh kg⁻¹, surpassing many state-of-the-art materials. Rate capability tests showed stable performance even at high currents, and full-cell experiments with graphite anodes maintained 70% capacity retention after 500 cycles. Operando XRD and XANES confirmed reversible oxygen redox without significant oxygen loss or irreversible phase transitions, distinguishing it from earlier Li-rich cathodes prone to rapid degradation. Theoretical modeling further validated that lithium migration within the layered structure activates oxygen redox in a reversible manner, suppressing structural collapse. Collectively, these findings demonstrate a viable path toward safe, cobalt-free cathode materials with both high energy density and long cycle life.
“Our study demonstrates that manganese-based, cobalt-free layered oxides can deliver high capacity and stability simultaneously,” said Seung-Taek Myung, senior author of the paper. “By carefully designing the O2-type structure, we stabilized the oxygen redox mechanism and avoided the pitfalls of irreversible oxygen release. This not only enhances safety but also ensures long-term performance. These insights provide a blueprint for developing next-generation cathode materials that are both cost-effective and environmentally sustainable, helping to reduce dependence on scarce and expensive cobalt.”
The success of this Mn-based, Co-free lithium-excess cathode has broad implications for energy storage technologies. Its combination of high energy density, long-term cycling stability, and improved thermal safety makes it particularly suitable for electric vehicles, grid-scale storage, and advanced portable electronics. By eliminating cobalt, the material also addresses sustainability concerns and reduces reliance on critical raw materials. Beyond immediate applications, the study provides mechanistic insights into cationic and anionic redox processes, guiding future cathode engineering. This research represents a significant stride toward safer, greener, and more powerful lithium-ion batteries essential for the clean energy transition.
References
DOI
10.1016/j.esci.2025.100376
Original Source URL
https://doi.org/10.1016/j.esci.2025.100376
Funding information
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2022R1F1A1063351 and RS-2024-00446825). Experiments at PLS-II were supported in part by MSIT and POSTECH. K. Köster and P. Kaghazchi gratefully acknowledge the computing time granted through JARA-HPC on the supercomputer JURECA at Forschungszentrum Jülich under Grant No. jiek12 and funding from the “Deutsche Forschungsgemeinschaft” (DFG, German Research Foundation) under Project No. 501562980.
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.